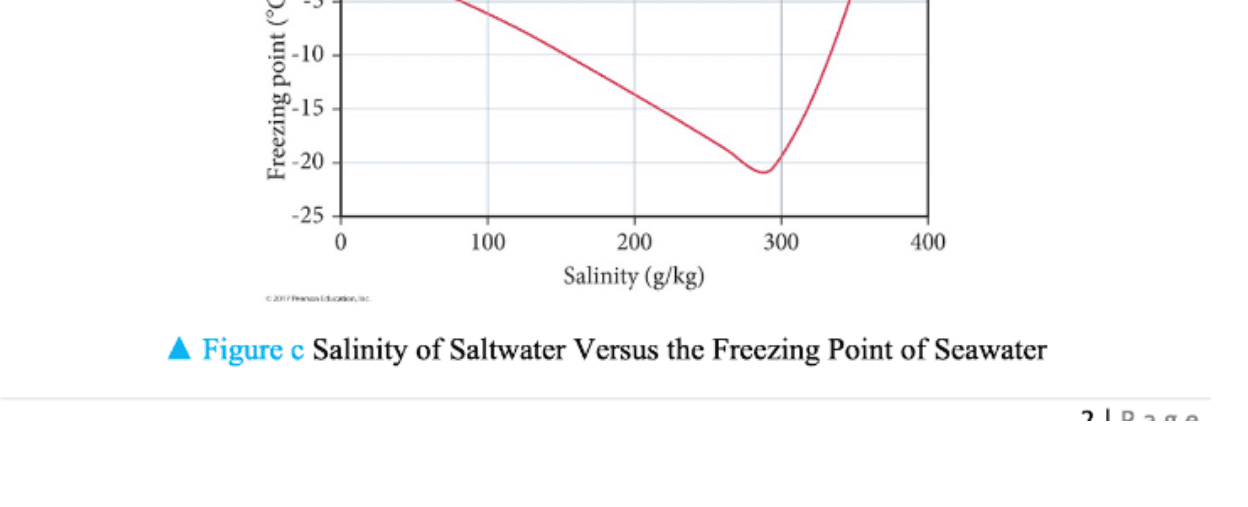
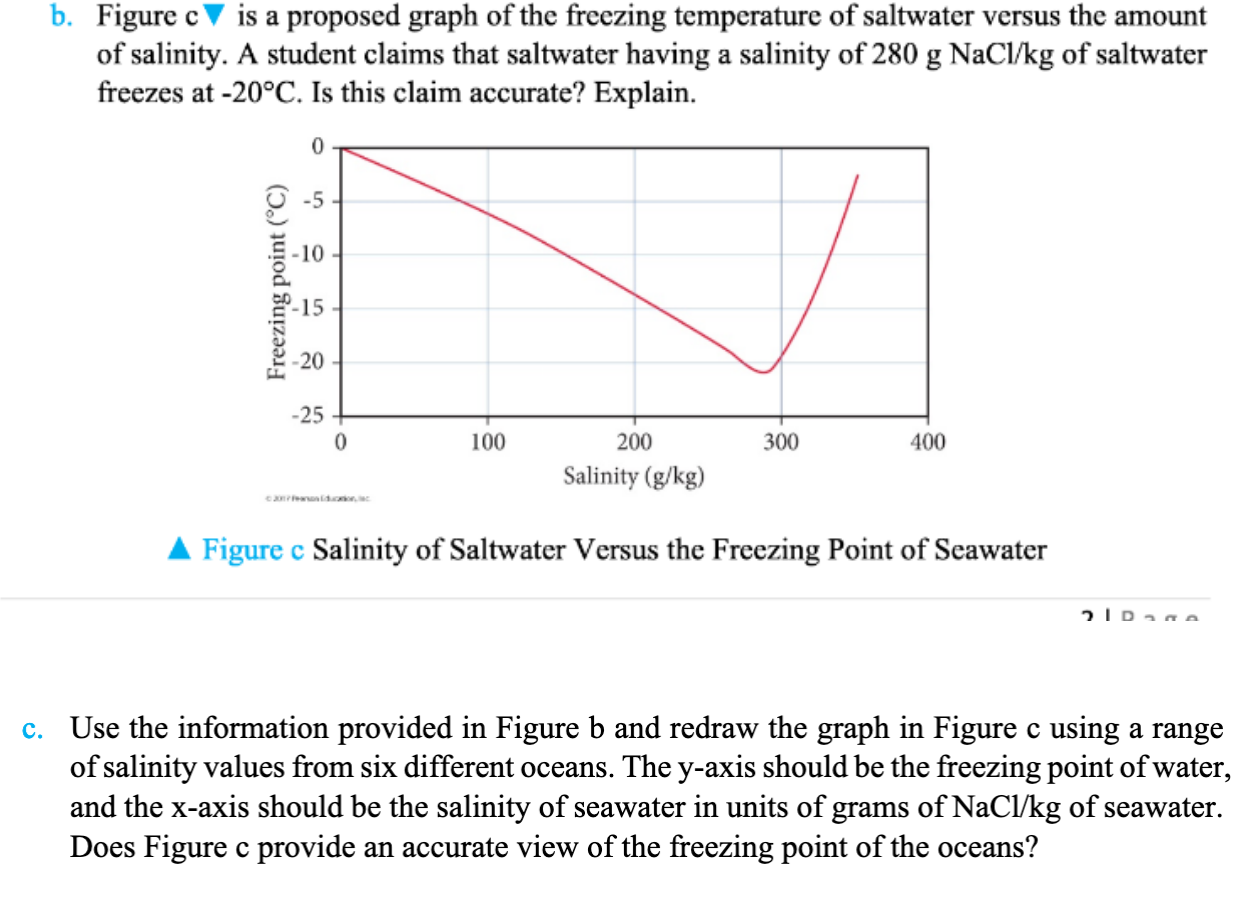
When seawater or saltwater freezes only the water part freezes its salt molecules are forced below the ice surface that s why polar ice caps are freshwater
When seawater or saltwater freezes, only the water part freezes
Have you ever wondered what happens when seawater or saltwater freezes? It’s a fascinating process that has a unique outcome. When temperatures drop low enough for freezing to occur, saltwater undergoes a transformation that separates its components.
Contrary to what some may think, when saltwater freezes, only the water part freezes. The salt molecules do not freeze but are rather forced below the ice surface. This phenomenon is due to the lower freezing point of saltwater compared to freshwater. The higher concentration of salt lowers the freezing point of the water, allowing it to remain in its liquid state at temperatures below the freezing point of pure water.
The salt molecules are forced below the ice surface

As the water freezes, the salt molecules become more concentrated in the remaining liquid water. This concentrated brine is denser than the surrounding freezing water and sinks. As a result, the salt molecules are forced downward, below the ice surface, while the freshwater forms the ice above. This process helps to create the unique composition of polar ice caps, which are mostly freshwater ice despite being surrounded by salty oceans.
The reason behind freshwater polar ice caps
The formation of freshwater polar ice caps can have significant implications for our planet. As climate change accelerates, the melting of these ice caps contributes to rising sea levels, leading to potential environmental and ecological challenges. Understanding the underlying mechanisms and characteristics of these ice formations is crucial in monitoring and predicting the impacts of climate change.
To delve deeper into this topic, you can explore the Wonderopolis article on saltwater freezing. It provides an insightful explanation on the freezing process and the behavior of salt molecules when water turns into ice. It’s a valuable resource to enhance your knowledge and quench your curiosity.
Remember, the next time you encounter polar ice caps, you can appreciate their unique freshwater composition even though they are surrounded by saltwater. The intricate balance between freezing temperatures and the behavior of salt molecules beneath the ice surface creates this awe-inspiring natural phenomenon.
Related Posts
Quick Links
Legal Stuff

