Water expands when it freezes ice has a lesser density than water so an ice cube takes up nearly 9 more volume than the water you used to make it
Water Expands When It Freezes: Understanding the Science Behind it
Water is a unique substance that exhibits various intriguing properties. One such remarkable fact is that water expands when it freezes. This is an exceptional characteristic, as most substances tend to contract when transitioning from a liquid to a solid state. Have you ever wondered why ice occupies more space than the water it was formed from? Let’s delve into the science behind this fascinating phenomenon.
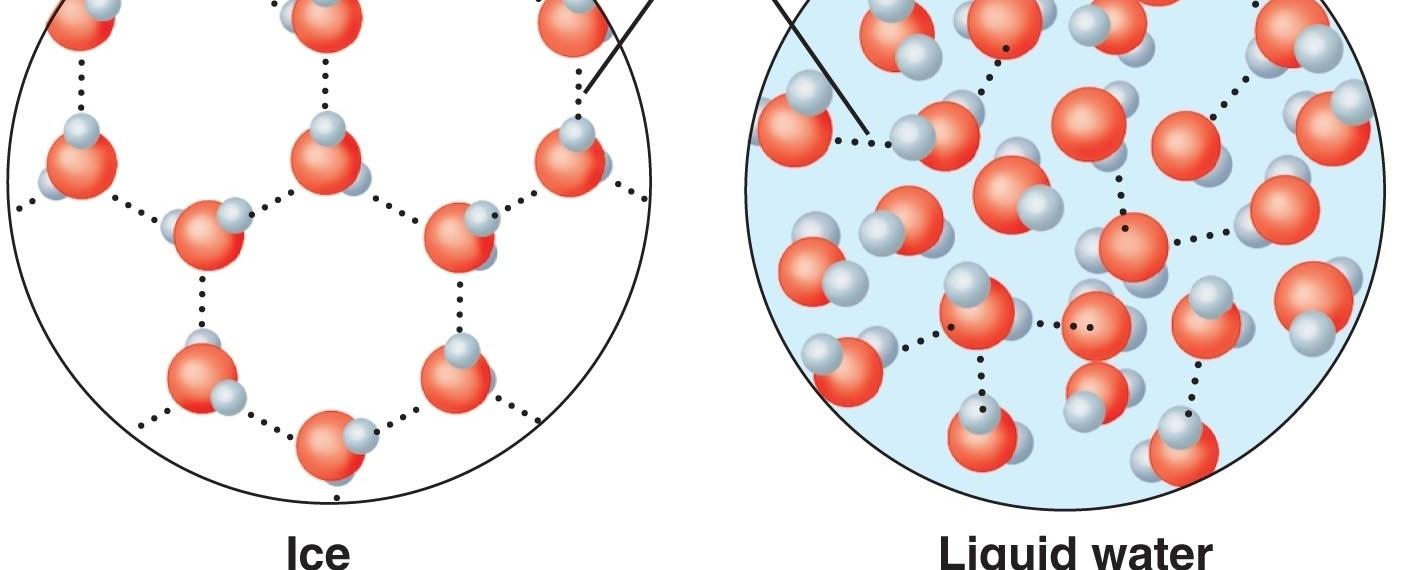
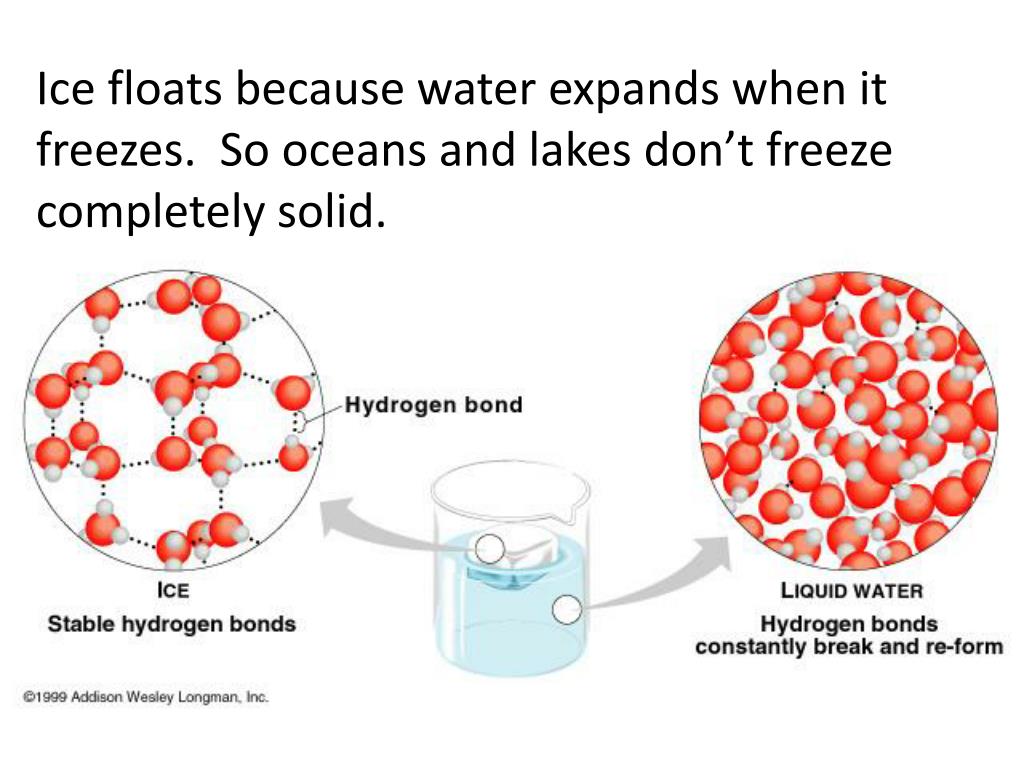
At the molecular level, water is composed of two hydrogen atoms bonded to one oxygen atom, resulting in its chemical formula H2O. Due to its polarity, water molecules arrange themselves in a particular pattern, forming a hexagonal structure when in a solid state, commonly referred to as ice.
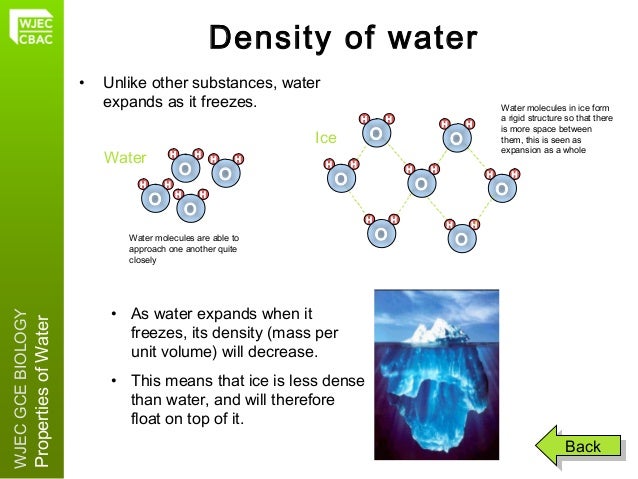
When water is cooled below its freezing point (0 degrees Celsius or 32 degrees Fahrenheit), these molecular bonds begin to strengthen and become more stable. As a result, the water molecules move further apart from each other, increasing the overall volume of the substance. This expansion occurs in all three dimensions, causing ice to be less dense than liquid water.
It is interesting to note that this expansion is not common among most substances. Generally, when a liquid transforms into a solid, its molecules arrange themselves in a more organized and compact manner, leading to a decrease in volume. However, water defies this norm due to its hydrogen bonding and specific molecular structure.
So, how much does an ice cube expand compared to its liquid counterpart? The expansion volume is approximately 9%, meaning that an ice cube takes up nearly 9% more space than the water used to create it. This expansion can be easily observed when freezing water in ice cube trays or when lakes and rivers freeze during colder seasons.
This unique property of water has significant implications for life on Earth. Without the expansion when freezing, bodies of water would freeze from the bottom up, making it extremely challenging for life forms to survive in aquatic environments. The fact that ice floats on the surface of water acts as an insulating layer, preserving life underneath it.
In conclusion, the phenomenon of water expanding when it freezes is not only intriguing but also crucial for the existence of life on our planet. Understanding the science behind this exceptional property allows us to appreciate the complexity and versatility of water. So next time you cool down your beverage with ice cubes, remember the remarkable nature of water and how it defies the norm by expanding when it turns into ice.
Source: Science Facts: Why Does Water Expand When It Freezes?
Related Posts
Quick Links
Legal Stuff

