Glass is easily breakable because of its amorphous loosely arranged atoms when colliding with a hard surface its atoms can t rearrange themselves as efficiently to maintain their structure therefore it breaks

Glass: Fragility Explained
Glass, a material known for its shiny and translucent appearance, has fascinated humans for centuries. From the majestic stained glass windows in ancient cathedrals to the delicate glassware we use in our daily lives, it is a versatile substance with countless applications. However, this remarkable material possesses a fragility that has intrigued scientists and researchers for years.
The reason behind glass’s brittleness lies in its atomic structure. Unlike crystalline solids, such as salt or diamonds, glass is amorphous, meaning its atoms are arranged in a disordered and loosely packed manner. This arrangement creates an inherent vulnerability, making glass prone to breaking when subjected to impacts or stress.
When glass collides with a hard surface, the loosely arranged atoms struggle to rearrange themselves effectively to maintain the material’s structure. In a crystalline solid, atoms are organized into a regular lattice, providing a more stable framework. However, in glass, the absence of this organized structure means that upon impact, the atoms cannot easily shift or adjust position to absorb the energy of the collision. Consequently, the force concentrates on a specific area, leading to the fracture and fragmentation of the glass.
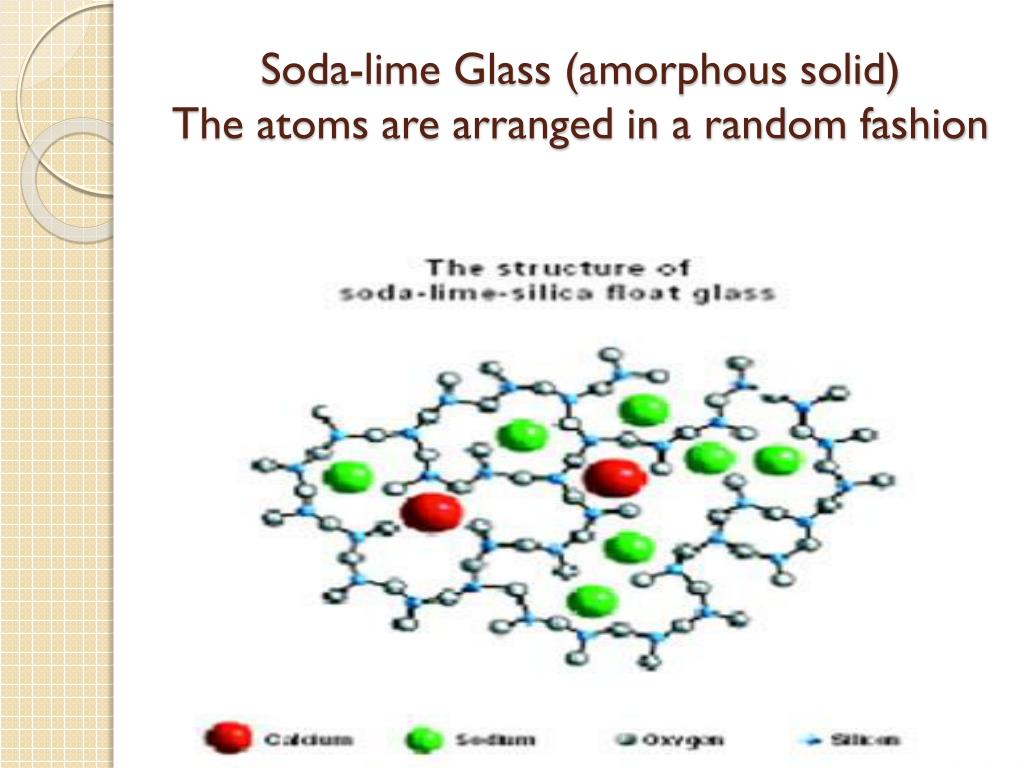
The vulnerability of glass to breakage has both scientific and practical implications. Understanding the mechanisms behind glass fracture is crucial for designing stronger and more durable glass products. By comprehending the limitations of glass, scientists and engineers can develop innovative techniques to enhance its strength and impact resistance.
Despite its fragility, glass remains highly sought after due to its exceptional qualities. Its transparency enables us to experience a connectedness with the outside world, and its ability to be molded into various shapes makes it a versatile medium for artistic expression. Glass’s brittleness also makes it a material of choice for safety purposes, such as in car windshields, as it shatters into small, less harmful fragments upon impact.
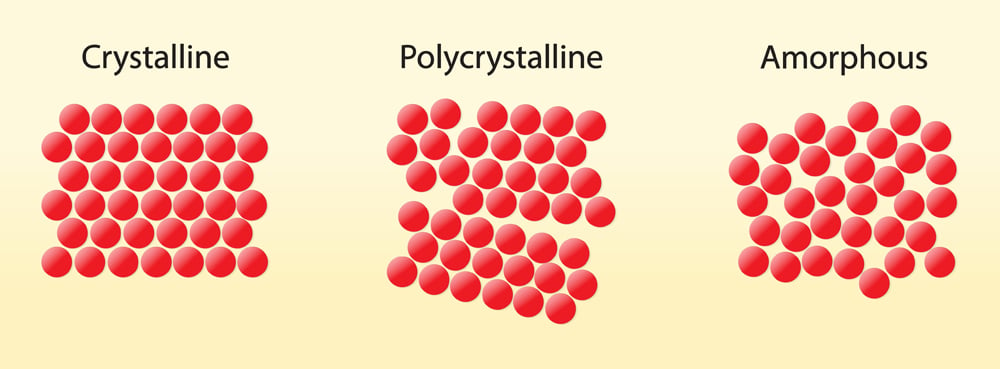
In conclusion, the amorphous and loosely arranged atomic structure of glass renders it susceptible to breakage when met with external forces. As the atoms are unable to efficiently rearrange themselves upon collision, the integrity of the material is compromised, resulting in fractures. Nonetheless, the fragility of glass also contributes to its unique appeal and functionality in various aspects of our lives.
Source: The Corning Museum of Glass
Related Posts
Quick Links
Legal Stuff

