What gas makes soda bubbly carbon dioxide
What gas makes soda bubbly? Carbon Dioxide.

When it comes to enjoying a refreshing soda, one of the most delightful aspects is the effervescence that tickles your taste buds. That bubbly sensation we all love is created by a gas called carbon dioxide (CO2). This fact, although simple, holds great significance in the world of carbonated beverages.
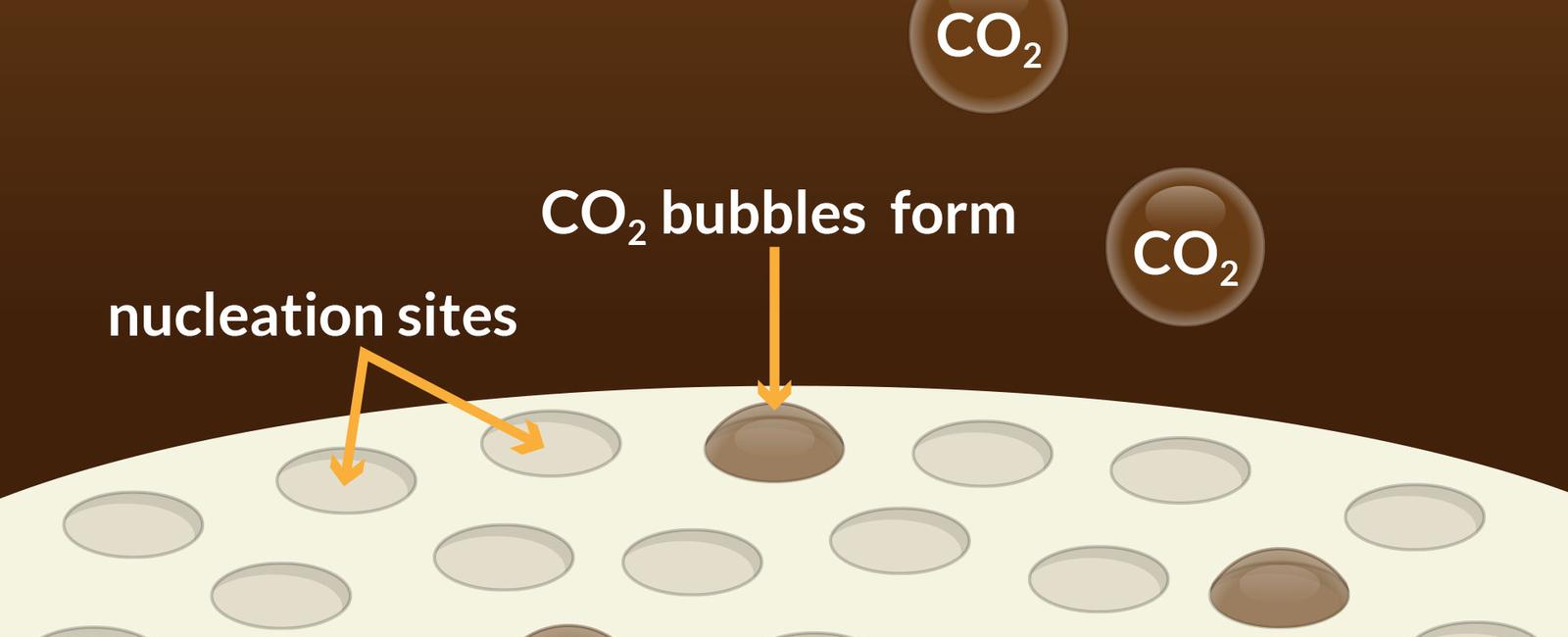
Carbonation is a process that involves the dissolution of carbon dioxide gas in a liquid, typically water. The result is the formation of small bubbles that dance their way to the surface and provide the characteristic fizziness we associate with soda.

So, how does carbon dioxide end up in our soda? Well, it all begins during the manufacturing process. When the ingredients for soda, such as flavorings, sweeteners, and water, are mixed together, carbon dioxide is injected into the solution at high pressure. This pressurized environment causes the gas to dissolve into the liquid, creating a solution that is supersaturated with CO2.
Once the carbonated beverage is bottled or canned, the carbon dioxide remains trapped within the liquid until it is released when the container is opened. When you crack open a can of soda, the sudden drop in pressure allows the carbon dioxide to come fizzing out of the liquid in the form of bubbles.
But what gives carbon dioxide this bubbly nature? Well, when CO2 dissolves in water, it chemically reacts to form carbonic acid (H2CO3). This acid then further dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). These reactions create an acidic environment and release carbon dioxide gas, which leads to the formation of bubbles.
During the consumption of carbonated beverages, the sensation of fizziness occurs due to the stimulation of our taste buds and nerves. The carbon dioxide bubbles that rise to the surface of the liquid burst upon contact with our tongues, generating a tingling and slightly acidic sensation. This experience adds to the overall enjoyment of consuming soda.
Aside from being responsible for the beloved bubbliness of soda, carbon dioxide also plays a crucial role in preventing microbial growth and preserving the freshness of the beverage. The gas acts as a natural preservative that helps extend the shelf life of carbonated drinks. Moreover, carbonation enhances the overall taste and mouthfeel, making sodas even more enjoyable.
In conclusion, it is carbon dioxide that fills our favorite sodas with delightful bubbles. Whether it’s the fizziness that excites our taste buds or the preservation properties it offers, CO2 plays a central role in the world of carbonated beverages. So, the next time you crack open a soda, take a moment to appreciate the carbon dioxide that brings that refreshing effervescence to life.
Tags
Share
Related Posts
Quick Links
Legal Stuff

