Water s naturally occurring physical properties and chemical composition make it the perfect universal solvent it mixes well with other substances thanks to its oxygen and hydrogen atoms polar arrangement
Water: The Perfect Universal Solvent
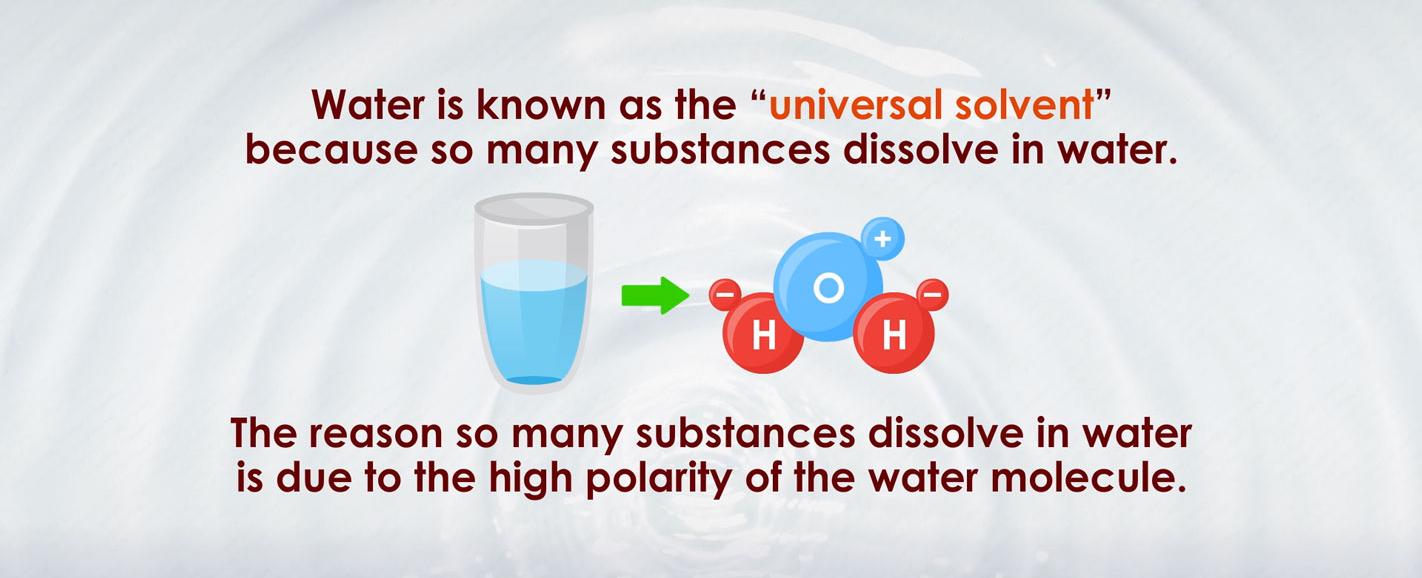
Water is a unique substance that plays a crucial role in sustaining life on Earth. Its naturally occurring physical properties and chemical composition make it the perfect universal solvent. But, have you ever wondered why water has such incredible solvent properties? Let’s delve deeper into the science behind it!
The Polar Arrangement of Water’s Molecules
Water consists of two hydrogen atoms and one oxygen atom, forming a molecule with a V-shaped structure. This arrangement results in a slight negative charge near the oxygen atom and a slight positive charge near the hydrogen atoms. Therefore, water molecules are polar.
The Importance of Polarity
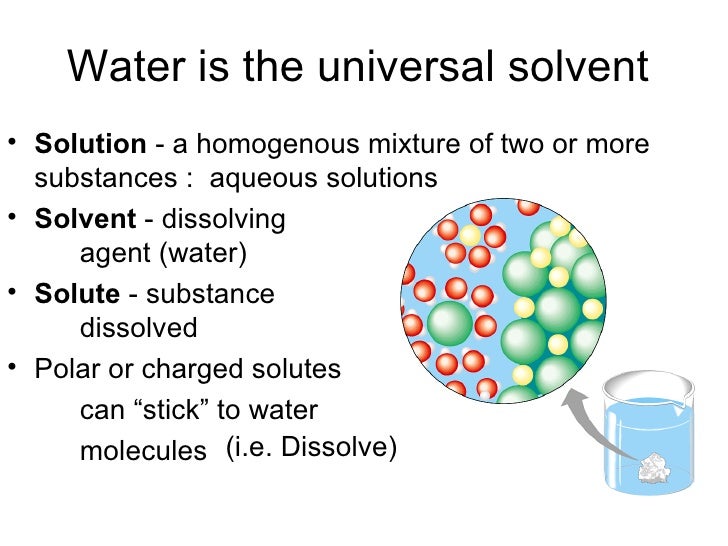
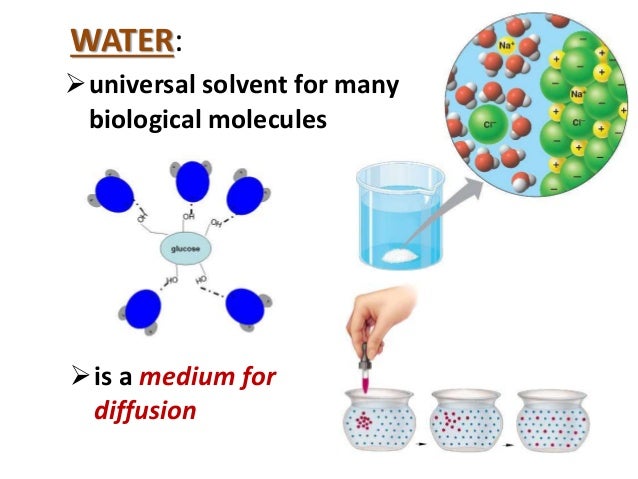
The polarity of water molecules plays a significant role in its solvent properties. Substances that can dissolve in water are known as solutes. Due to its polarity, water easily attracts and surrounds solute particles, helping them dissolve. This process is known as hydration or dissolution.
The Power of Hydrogen Bonding
Water’s polarity also enables hydrogen bonding, where the partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another water molecule. This bonding further enhances water’s solvent abilities. The hydrogen bonds between water molecules are weak individually, but collectively, they create a strong cohesive force.
Versatility as a Solvent
Thanks to its excellent solvent properties, water can dissolve a wide variety of solutes, including ionic compounds, polar molecules, and some nonpolar substances. This versatility is crucial for many biological and chemical processes. Water acts as a solvent in our body, transporting nutrients, eliminating waste, and regulating temperature.
The Dissolving Process
When a solute is introduced to water, water molecules surround the solute particles, gradually weakening the forces holding the solute together. Eventually, the solute fully dissolves, forming a homogenous mixture. Ionic compounds dissociate into their constituent ions, surrounded by water molecules.
Environmental Implications
Water’s universal solvent nature is of utmost importance for the planet. It enables the natural processes of weathering and erosion, breaking down rocks and minerals, and shaping the Earth’s surface over time. Moreover, water’s solvent properties facilitate the vital cycling of nutrients in ecosystems, ensuring the growth of plants and supporting aquatic life.
In conclusion, water’s naturally occurring physical properties and chemical composition make it the perfect universal solvent. Its polar arrangement, hydrogen bonding, and versatility in dissolving various solutes contribute to its unique capabilities. Understanding water’s solvent properties helps us appreciate its significance in sustaining life and shaping our planet.
Source: USGS Water Science School
Tags
Share
Related Posts
Quick Links
Legal Stuff

