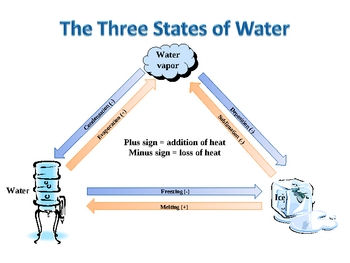
Water can exist in three states at once this is called the triple boil and at that temperature water exists as a gas a liquid and a solid simultaneously
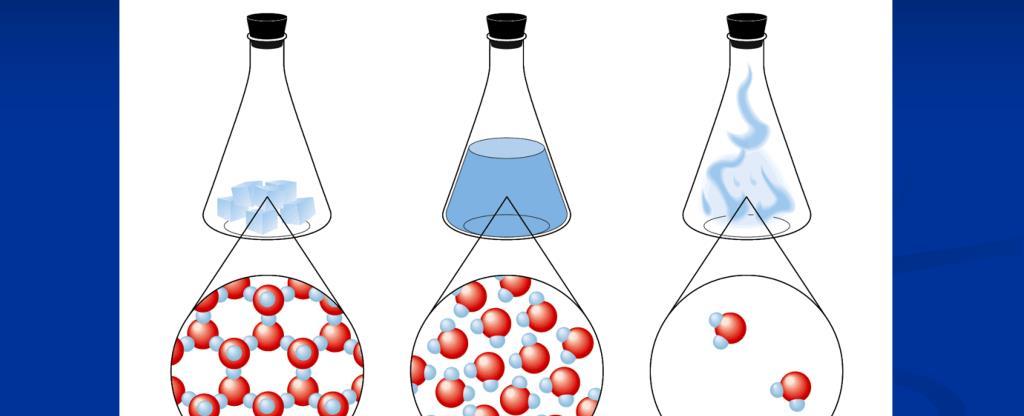
Water: The Incredible Triple Boil
Water is an essential and fascinating substance that we encounter on a daily basis. We all know that water can exist in three different states: solid, liquid, and gas. But did you know that it is actually possible for water to exist in all three states simultaneously? This mind-boggling phenomenon is known as the Triple Boil.
At a certain temperature, referred to as the Triple Boil point, water defies conventional expectations and transitions into a truly remarkable state. The Triple Boil occurs when water simultaneously exists as a solid, a liquid, and a gas. This incredibly rare event challenges our perception of how matter behaves and opens up a whole new world of possibilities in scientific understanding.
To fully grasp the magic of the Triple Boil, we must delve into the individual states of water. By utilizing the well-known states of water – solid, liquid, and gas – scientists have managed to create an intricate diagram that maps out the various conditions under which water can exist.
Imagine if you will, a diagram showing a triangular area where the solid, liquid, and gas states of water converge. This is precisely where the Triple Boil occurs. As the temperature rises, the boundaries between the states of water become blurred until they merge into a single point. At this point, the Triple Boil point, water miraculously takes on three simultaneous physical forms.
This extraordinary phenomenon has left scientists in awe as they attempt to unravel the mysteries of the Triple Boil. It challenges our understanding of the physical properties of matter and has significant implications for various fields of study, including thermodynamics and material science.
But how does this peculiar event take place? The secret lies in the delicate balance between temperature and pressure. When the temperature and pressure of water reach the precise conditions required, the molecules of water gain enough energy to freely move and transition between states, coexisting as a solid, a liquid, and a gas.
The Triple Boil serves as a testament to the intricate nature of water and its ability to defy expectations. Its existence illuminates our understanding of intermolecular forces and the delicate equilibrium required for such a phenomenon to occur.
In conclusion, the Triple Boil showcases the enchanting complexity of water. Through its simultaneous existence as a solid, a liquid, and a gas, water challenges us to explore the limits of our scientific knowledge. This awe-inspiring phenomenon unlocks new possibilities and leaves us marveling at the wonders of the natural world.
Sources:
Images:
Tags
Share
Related Posts
Quick Links
Legal Stuff