The amount of salt in the water impacts the temperature at which a sea will freeze however it will typically freeze around 28 degrees f 2 22 c
The Impact of Salt on Freezing Temperatures of Sea Water
Have you ever wondered why the sea doesn’t freeze completely in frigid temperatures? It turns out that the amount of salt in the water has a significant impact on the freezing point. While sea water will typically freeze around 28 degrees Fahrenheit (-2.22 degrees Celsius), the presence of salt lowers the freezing temperature compared to fresh water. In this article, we will explore the fascinating relationship between salt and freezing temperatures in sea water.
Saltwater is a mixture of water and various dissolved salts, with sodium chloride (common table salt) being the predominant salt. When water freezes, it forms ice crystals as the individual water molecules slow down and come together. However, the presence of salt disrupts the formation of these ice crystals and lowers the freezing point.

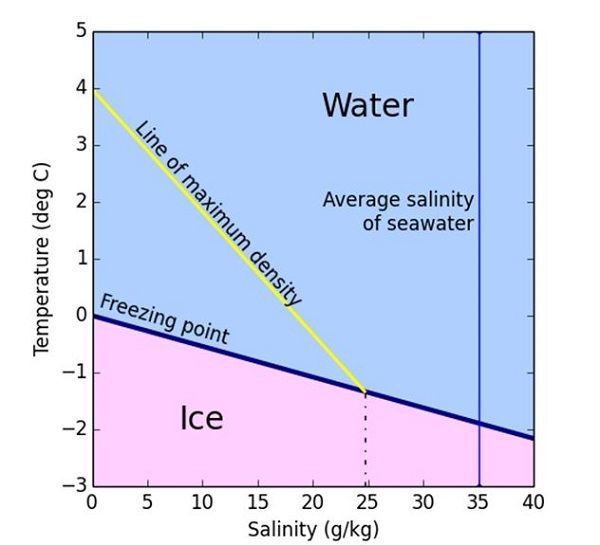
The freezing point of saltwater decreases as the salt concentration increases. This is due to the way salt molecules interfere with the arrangement of water molecules, making it more difficult for them to form the organized structure of ice. The salt ions create disorder within the water, preventing the formation of a solid lattice.
On average, the freezing point of seawater decreases by about 0.5 degrees Fahrenheit for every 3.5% increase in salt concentration. This means that the higher the salt content, the lower the freezing point. In extremely salty regions, such as the Dead Sea or the Great Salt Lake, water can remain liquid even at temperatures below freezing.
Understanding the impact of salt on freezing temperatures is crucial for various scientific disciplines. It helps oceanographers and climatologists study the formation and movement of sea ice, which has significant implications for global climate patterns and marine ecosystems. Additionally, it has economic significance for industries such as fishing and shipping, as the freezing point of seawater plays a role in determining navigability and formation of icebergs.
In conclusion, the amount of salt in seawater plays a critical role in determining its freezing point. While sea water will typically freeze around 28 degrees Fahrenheit (-2.22 degrees Celsius), the presence of salt lowers the freezing temperature. This intriguing relationship between salt and freezing temperatures is vital for understanding climate patterns and ocean dynamics.
Source: What Are the Seven Seas?
Tags
Share
Related Posts
Quick Links
Legal Stuff