Because of vulcanization a rubber tire is technically one big polymerized molecule charles goodyear who the tire company was named after discovered the process of crosslinking large polymer chains
The Fascinating Process of Vulcanization in Rubber Tire Manufacturing

Rubber, one of the most versatile materials known to humanity, has revolutionized various industries, primarily through its utilization in tire manufacturing. Surprisingly, rubber tires owe their integrity to a remarkable scientific process called vulcanization. It is due to this process that a rubber tire can be considered as a single, colossal polymerized molecule.
The Ingenious Discovery of Vulcanization by Charles Goodyear
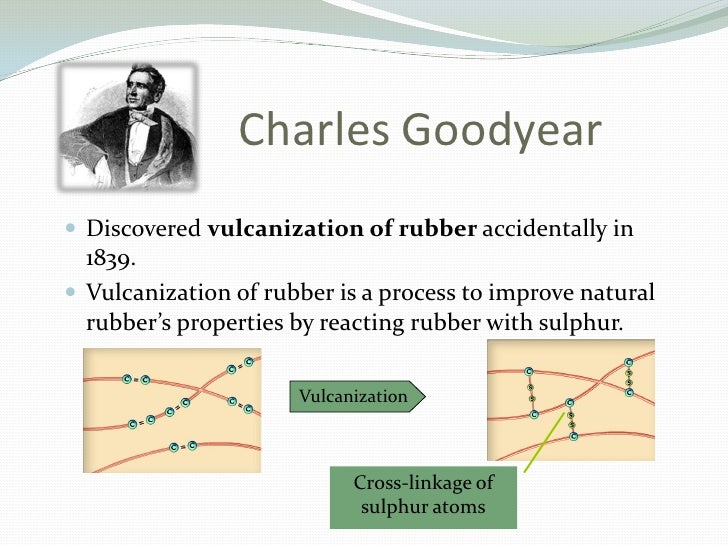
The history of vulcanization dates back to the early 19th century when Charles Goodyear, a renowned American chemist and inventor, made a groundbreaking discovery. During his tireless pursuit of enhancing the properties of raw natural rubber, Goodyear stumbled upon a process that resulted in a significant breakthrough - crosslinking large polymer chains. This transformative process, known as vulcanization, revolutionized the rubber industry, paving the way for the incredible durability and strength rubber tires possess today.
Unraveling the Marvels of Vulcanization
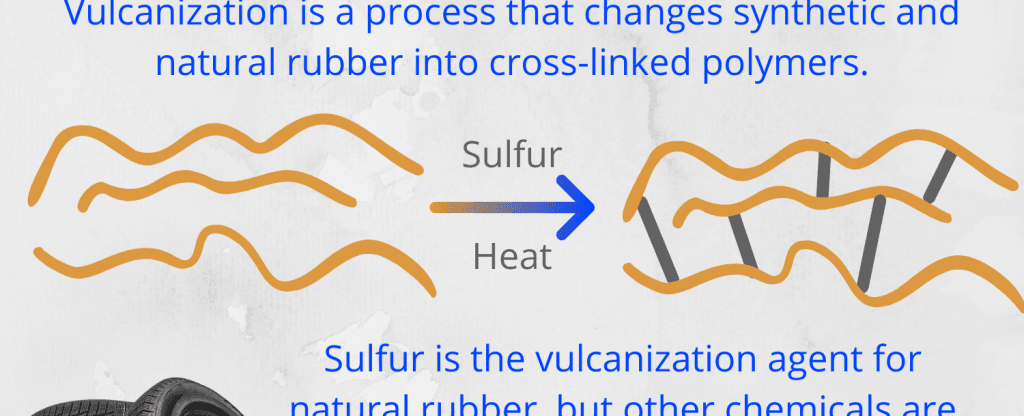
Vulcanization, in simple terms, involves treating raw natural rubber with specific chemical agents to modify its physical properties. These agents typically include sulfur and accelerators, such as organic compounds or metallic oxides. The process brings about a series of reactions that result in an extraordinary transformation of the rubber.
Firstly, the long polymer chains found in raw natural rubber are crosslinked or bonded together by sulfur atoms. These crosslinks create a three-dimensional network within the rubber matrix, enhancing its strength and resistance to wear and tear. It is this network of interconnected polymer chains that symbolizes the tire’s structure as a colossal polymerized molecule.
Secondly, the vulcanization process imbues the rubber with exceptional elasticity and flexibility. By forming stable chemical bonds, the rubber gains resistance to changes in temperature, thus enabling it to maintain its shape and performance, whether in scorching summers or freezing winters.
Additionally, vulcanization significantly reduces the rubber’s susceptibility to swelling, making it even more resistant to chemicals and solvents encountered on roads. This property enhances tire longevity and minimizes the occurrence of punctures or other forms of damage.
The Significance of Vulcanization in Tire Manufacturing
The discovery of vulcanization proved to be a game-changer for the tire industry. Prior to this remarkable breakthrough, natural rubber tires were plagued by numerous shortcomings, including poor heat resistance, low durability, and vulnerability to extreme weather conditions. However, with the advent of vulcanization, these limitations were unequivocally overcome.
Today, vulcanization is an integral part of the tire manufacturing process. As raw natural rubber is mixed with various additives and chemicals, it undergoes vulcanization to ensure optimal performance, longevity, and safety on the roads. Whether it’s heavy-duty truck tires, performance car tires, or bicycle tires, the application of vulcanization remains a crucial step in creating high-quality, reliable, and long-lasting rubber tires.
In conclusion, the tire industry owes its success and endurance to Charles Goodyear’s groundbreaking discovery of vulcanization. Through the process of crosslinking large polymer chains, rubber tires are transformed into one immense polymerized molecule, enveloping them with strength, durability, and resilience. It is this extraordinary process that enables rubber tires to withstand the demands of various road surfaces, weather conditions, and intensive usage while indeed going the extra mile.
Source: PolySciLinux
Tags
Share
Related Posts
Quick Links
Legal Stuff